NoCo Science Education Blog: Decades of Discovery – Explorations with Dr. Stephen Thompson
By Victoria Jordan

Dr. Stephen Thompson, Explorer
Microtornadoes, microstalagtites, and microhurricanes are beautiful 3-dimensional gaseous structures created by chemical reactions in Dr. Stephen Thompson’s laboratory. In order to facilitate his explorations of molecular fields, Dr. Thompson built microscopes himself that include high resolution video with fiber optic connectors and various lasers to study chemical reactions on the fly as they take place.
Microscopes

He is exploring anthocyanins (colorful plant pigments) using spectroscopy and chromatography to find mathematical patterns. He is conducting microfluidic electrophoresis of anthocyanins by taping sewing pins onto a microscope slide so he can watch and film the process as it happens under the microscope.

Dr. Thompson is using capillary tubes to view aerosols for experiments with colored lasers, transferring large scale experiments to small scale where the observations can take place on a molecular level. His passion for exploration is never-ending.
Small Scale Chemistry
Dr. Thompson is best known for his groundbreaking development of Small-Scale Chemistry to promote student-centered inquiry in college level chemistry classes. In the 1990’s, he co-wrote a grant to create the Center for Science, Mathematics and Technology Education (CSMATE) at Colorado State University, and helped to design a modern college experiential studio where he utilized Small Scale Chemistry methodologies that encouraged students to learn science by doing science. Thompson shared his educational philosophy through the lens of Small-Scale Chemistry to K-16 public school teachers by offering numerous professional development courses funded by the National Science Foundation, the Department of Energy, the Environmental Protection Agency and others. The collaborative partnerships’ that Thompson nurtured with public school teachers helped to transform the way an entire generation of science teachers and their students experienced learning and exploring science.
Tim Lenczycki, a high school chemistry teacher, is one of many teachers that have adopted small scale practices. Tim says, “Dr. Thompson is not only a spell-binding lecturer, but his expertise as an educator really shines one-on-one. He truly finds importance in student opinions and honestly values their insights. He empowers students in their own knowledge and skillfully leads them to higher levels of understanding. I have not met a person who is more well-read than Steve Thomson. Coupled with his vastly varied other pursuits, he is an authentic renaissance man.”
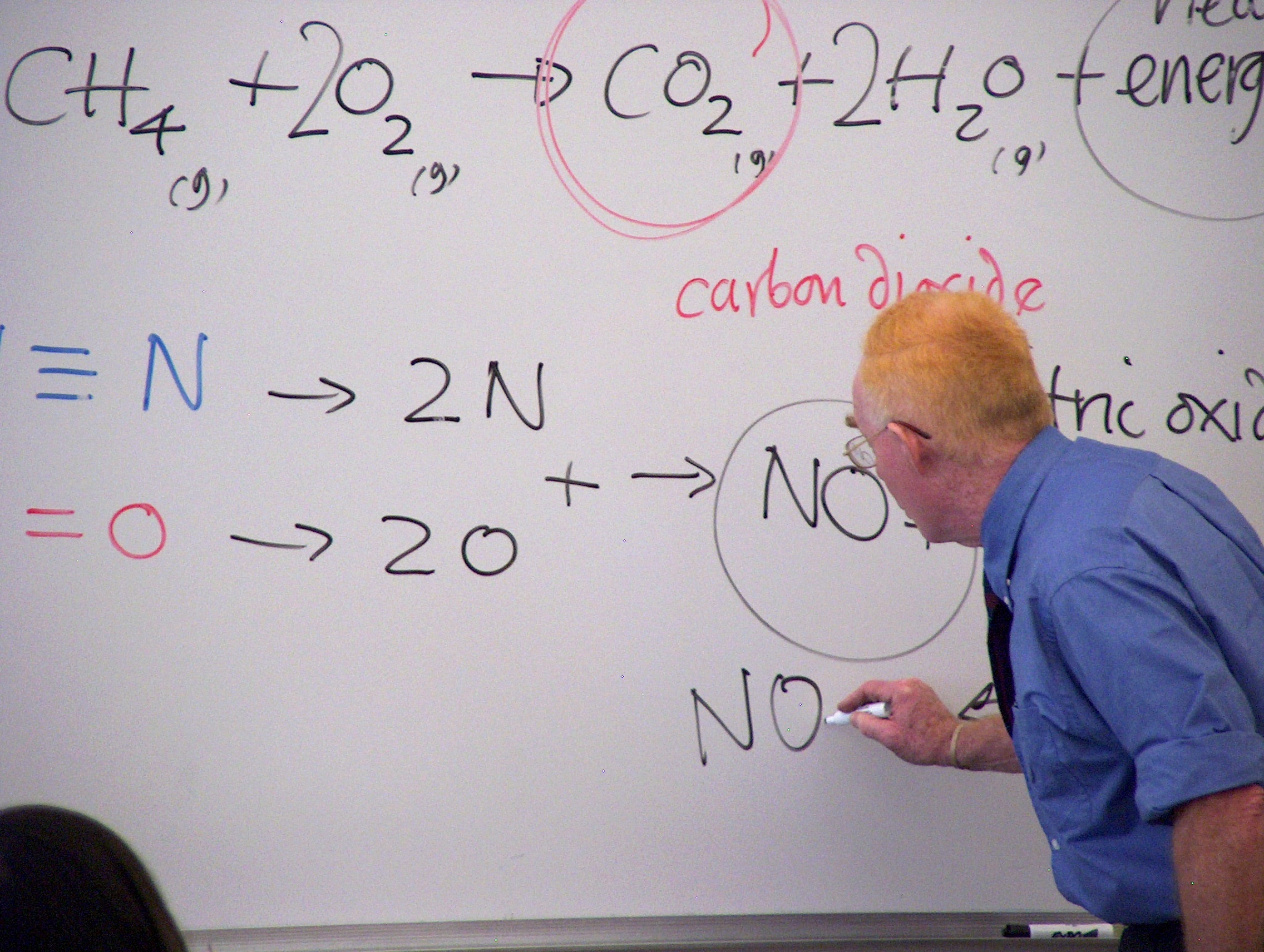
Dr. Thompson attempted to influence University science education away from the business model where hundreds of students can be packed into a lecture hall to more of an experiential model in which students could scientifically explore natural patterns with guidance from the instructor. In Thompson’s model the teacher takes on the role of mentor, thus learning becomes a partnership with his students. His college text, ChemTrek, has been translated into several languages, and is used extensively in Asia. Thompson has carried out workshops in Korea, Japan and Thailand to broadcast small scale approaches across the globe.

The value of Thompson’s Small-Scale Chemistry methodologies are even embraced by schools that have not had the opportunity to learn his student-centered pedagogy. “Asian schools doing chemistry who were using traditional methods of teaching were generating large amounts of hazardous waste, and they adopted Small Scale Chemistry in order to solve some environmental problems they were having with hazardous waste management in schools and universities. It also happens to save millions of dollars,” Steve says. Small Scale Chemistry allows every student to design and carry out their own experiments using multiple iterations, partly because there is little waste and a huge cost savings. Additionally, Small Scale Chemistry is much safer than traditional chemistry labs that use large glassware and high volumes of potentially dangerous chemicals. Dr. Thompson earned recognition for eliminating accidents in the lab during his tenure at CSU.

Every teacher who has gone through one of Steve’s trainings takes away the importance of student exploration and engagement. A Thompson lesson typically starts with introducing students to a natural phenomenon, which is followed by guided exploration and finally open inquiry. This process nurtures a student’s ability to discover natural patterns by asking questions and designing experiments. The hands-on approach also encourages students to think critically about what they claim as knowledge. “How do you know that?” is one of Steve’s mantras. Getting students to think, to ask questions, and to try to find answers is his forte. “Small scale isn’t just about reducing the size of the experiment. It’s about how teachers and students approach the learning.”
On the Shoulders of Giants
Dr. Thompson surrounds himself with inspiration, and has a very extensive private scientific library. “I am a student of the history and philosophy of science,” he says. “I have every book written on the history of laboratories, and I have an incredible collection of books regarding women in science, astronomy, biology and chemistry. Scientists are people with stories, and I want to know who these people were.” For example, a German scientist, Rafael Liesegang, wrote a paper in 1896 about diffusion gels. Thousands of people reference this work, but very few have ever studied it directly. During the Covid lockdown, Steve translated all 53 pages from the original German to English. Thompson and colleague Dr. Patrick Shipman are extending Liesegang’s work and developing mathematical models for the pattern systems from that original work.

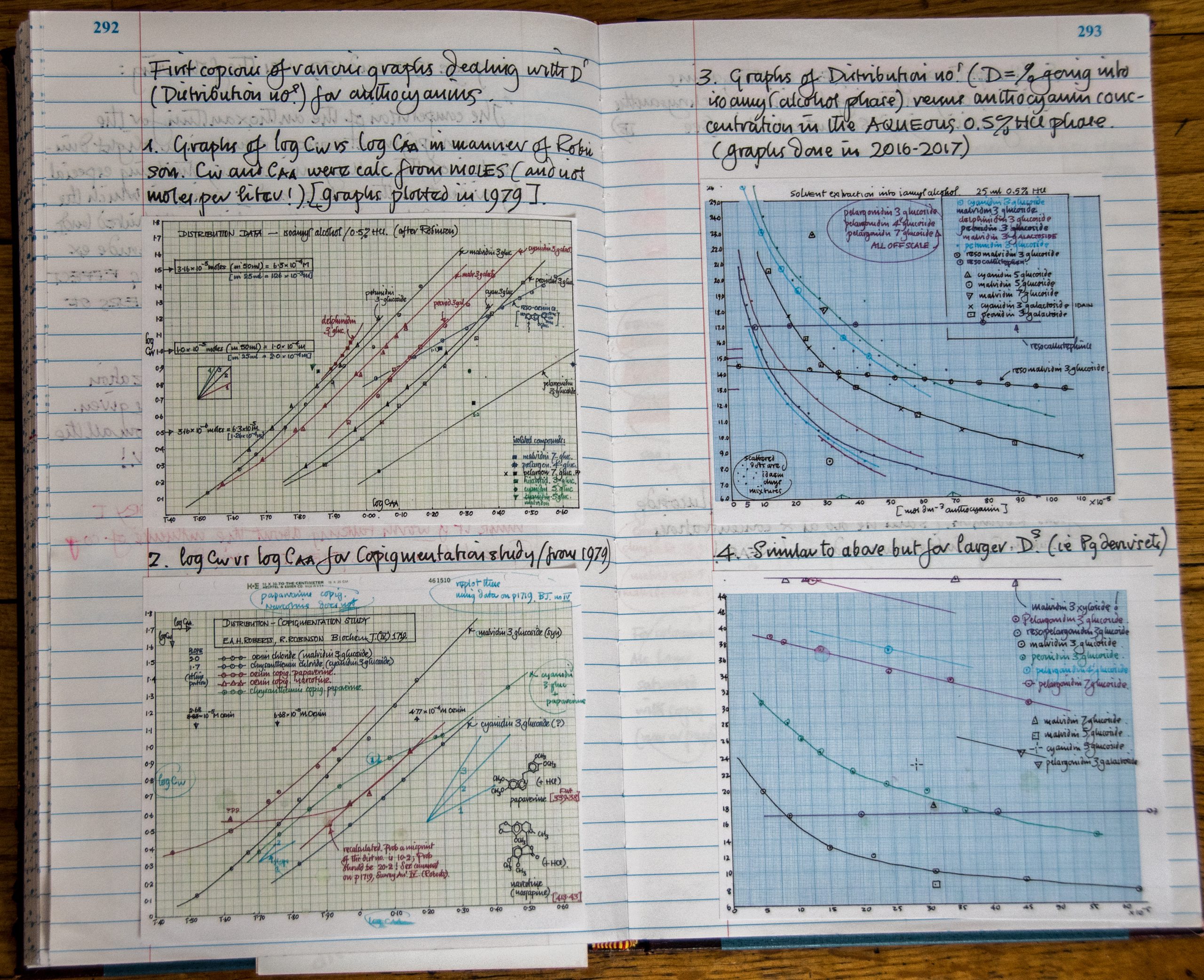
When Steve looked at the literature from the 1890’s regarding the chemistry of anthocyanin pigments, he analyzed the works of 2 Nobel scientists: British organic chemist, Sir Robert Robinson and a German chemist, Richard Willstatter. Steve found that Willstatter grew flowers by the acre in Berlin and extracted the anthocyanins to analyze their structure. Steve is exploring the concept that anthocyanin associations influence flower color, odor and pollination ecology, and has assisted graduate students including Dr. Wei Yu who recently completed a thesis project on this topic. Steve has taken all the data he can find in the literature, plus his own data, and is coming up with new conclusions and new experiments.
Steve’s work on teaching the chemistry of climate change has been inspired by his favorite chemist, John Tyndall. In an 1880’s book Heat: A Mode of Motion, Tyndall explored climate change by examining the transmission of energy in the form of heat. Steve has taken Tyndall’s original experiments and is translating them to a small-scale version using micro-encapsulated liquid crystal mylar systems to model and explore energy transfer.
Dr. Thompson is never alone in his lab. He always brings scientists past and present into his work. Currently he is working with 2 graduate students as they complete research projects. One is examining the fundamental chemistry of why sinkholes are forming in Horsetooth Reservoir. The other is exploring anthocyanins as phytophoto protection for newly forming organelles in plants. Dr. Thompson loves being a researcher and a teacher, and has no plans to stop doing either.

From Then to Now
Born in a small mining town in northern England, Dr. Thompson earned his PhD from the University of Birmingham, England. From there, he dropped out of academia to grow bananas and play tennis on St. Vincent in the West Indies. When he decided to re-enter society, he came to America on a post-doc fellowship to the University of Arizona in Tuscon, and then became an Assistant Professor at the Case Institute of Technology in Cleveland. When a professor at Colorado State University saw him eating fire at a demonstration in 1969, he reached out and said, “I think you would be a great teacher in our freshman courses,” and Steve never looked back.

Dr. Thompson claims his methods are not special. “If you want to find out what’s going on, you have to start with what you know. All we do in science is take something we don’t know anything about and put it next to something we know something about and find the similarity.
The history of science is really the history of containers,” he claims. When he first started teaching chemistry at CSU, everyone was using large amounts of chemicals in large containers to prove a concept. “Now that we are working on the molecular level to explain and prove concepts, we can use very small amounts of materials in very small containers. Take a drop of water. In that drop, there are billions of molecules to study!” Steve exclaims.

True to his nature, Thompson studied another English scientist, Michael Faraday. Faraday viewed chemical reactions as molecular fields in the early 1800’s. Small Scale Chemistry came out of the methodologies Faraday used as an experimenter, and Steve’s perusal of Faraday’s notebooks helped him create his methods. A S3TAR was born. The S3TAR (Small Scale Science- Teachers As Researchers) program brought together middle and high school science teachers to learn Small Scale methodologies in summer programs, and those teachers went on to train their departments, influencing an entire generation of science teachers.

The Future
Smart Scale Sustainable Science and Mathematics (S4M) is Steve’s latest iteration of how he would like to see science and math taught in schools. “The walls that separate the traditional sciences are beginning to crumble. The compartmentalization of the disciplines needs to go away, and mathematics, the language of science, is the thread that ties them all together.”
The pandemic brought to light all the failings of the educational system, and Thompson is currently researching better ways to get students to learn, to explore, and to embrace science and math. “The fundamental problem is that most classrooms are lecture-based. We need to infuse phenomena and exploration into every lesson. Experimental work is disappearing in classrooms, and I am hopeful that S4M can do away with lecture and provide ways for 100% of the students to engage in explorations of their world 100% of the time.” He is working on a book with the working title of Existential Education. In his 60 years of investigating chemistry and 40 years in education, Steve has continued to grow, explore and learn while inspiring teachers and students to do the same.
Stay tuned, as Dr. Stephen Thompson’s Opus has yet to be concluded.
See more of Dr. Thompson’s work at these links:
Thompson and Shipman’s paper in the Journal of Chemical Physics on the counterdiffusion of HCl and NH3

